Efficient sheep reproduction is vital in modern livestock farming, particularly in breeding programs aimed at genetic improvement and embryo transfer. One of the most critical techniques in managing and evaluating reproductive performance is ultrasonography. Among its many applications, ultrasound imaging provides valuable insights into the growth and development of ovarian follicles in ewes undergoing superovulation. This article explores the key ultrasound features of follicular development in sheep, discusses the ideal scanning time, and explains why this technique is gaining widespread adoption globally.

Understanding Follicular Development in Ewes
Ovarian follicular development in sheep follows a dynamic process that begins with the emergence of small antral follicles and progresses to the selection and dominance of larger follicles. Each follicle contains fluid—known as follicular fluid—and surrounds an oocyte (egg). Monitoring the growth of these follicles during a superovulation protocol is crucial for optimizing timing in artificial insemination or embryo collection.
In natural and hormonally induced cycles, follicles grow in waves, with dominant follicles reaching ovulatory size under the influence of gonadotropins such as follicle-stimulating hormone (FSH). The use of ultrasonography—specifically, Modul B (brightness mode) ultrasound—has become the gold standard for visualizing this process in a non-invasive and repeatable manner.
Key Ultrasonographic Features of Ovarian Follicles
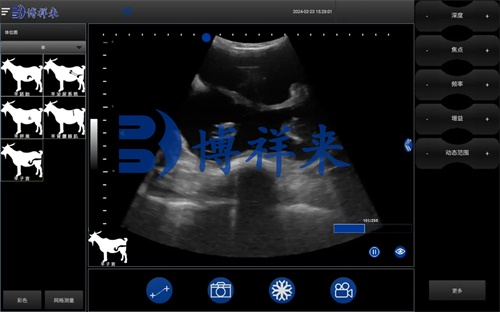
Ultrasound images of follicles are characterized by their fluid content, which appears as anechoic (black) regions on the monitor. These anechoic zones are typically surrounded by a bright, echogenic rim representing the follicle wall. The contour of a healthy growing follicle is smooth and round, indicating normal development.
During the early stages of superovulation—especially one day before FSH administration—sheep ovaries often show a homogeneous parenchymal texture with scattered small follicles, typically Mai puţin 2 mm in diameter. These follicles appear as tiny, dark fluid pockets on the screen, located mostly at the ovarian periphery.
Once superovulation is initiated (usually through the administration of FSH), ultrasound is used to monitor the ovarian response:
-
Day 1 of Superovulation: After two 4 mL FSH injections (morning and evening), the ovaries may appear enlarged, but individual follicles are not clearly increased in size.
-
Day 2: After another pair of 3 mL FSH injections, the ovaries show further enlargement, and the uterine wall thickens, indicating systemic hormonal response. Însă, follicles still remain under 2 mm.
-
Day 3 (Morning): Small follicular clusters become visible again, and by the afternoon, several follicles begin to enlarge, though most still remain below 5 mm.
-
Day 4: This is a critical time point. The ovaries now appear significantly enlarged, and many follicles reach 5–8 mm in diameter. The ultrasound displays numerous clear, round, dark fluid-filled structures—each representing a mature follicle poised for ovulation.
By slowly moving the ultrasound probe across the ovary, veterinarians can track changes in follicle location, formă, and size, as well as estimate the total number of developing follicles, particularly those that protrude from the ovarian surface.
International Insights and Application in Modern Sheep Farming
In countries like Australia, New Zealand, and the United Kingdom—where sheep farming plays a significant economic role—ultrasound monitoring has become an integral part of reproductive management. According to veterinary researchers in Europe and North America, follicular ultrasonography is used not only to track superovulatory response but also to select optimal donor ewes in embryo transfer programs.
An increasing number of livestock technicians are being trained in reproductive ultrasonography, and portable scanners like the BXL-V50 and similar Ecografie veterinară units are now commonly used in the field. Their durability, long battery life, and waterproof design make them ideal for farm use.
Ultrasound is particularly helpful in determining when to cease hormonal stimulation and proceed to artificial insemination. In research from the University of Saskatchewan (Canada), it was shown that timing insemination based on follicular size and maturity via ultrasound resulted in higher conception and embryo recovery rates compared to fixed-time protocols.
Best Time to Perform Ultrasonography During Superovulation
While daily monitoring offers detailed insight, studies and on-farm experiences indicate that the most informative period for follicular ultrasound in ewes is between Day 3 and Day 4 after initiating superovulation.
-
Day 3: The initial signs of follicle growth become evident, and variation among ewes in response to hormone treatment starts to appear.
-
Day 4: The follicles reach their peak size and number, making it the ideal window to evaluate superovulatory response and plan insemination.
Beyond Day 5 or Day 6, most donor ewes will enter estrus and begin ovulating. At this stage, continued ultrasound monitoring is generally discouraged, as repeated handling and scanning can cause stress, potentially disrupting ovulation or impacting the function of the oviductal infundibulum (the funnel-like opening of the oviduct that captures the ovulated egg).

Advantages of Ultrasonography in Follicle Monitoring
Sheep breeders around the world now recognize the value of ultrasound in reproductive success. Its key advantages include:
-
Non-invasive: No surgery or internal probes are required; a transabdominal or transrectal probe provides sufficient imaging.
-
Real-time imaging: Helps visualize follicle dynamics as they happen.
-
Accurate follicle count: Allows estimation of how many viable oocytes might be collected during embryo flushing.
-
Improved breeding decisions: Enables better planning for artificial insemination or embryo transfer based on follicle maturity.
-
Stress-free monitoring: When performed correctly, it has minimal impact on animal behavior or physiology.
Technological Progress and Global Adoption
The growth in veterinary imaging technologies has also played a crucial role in the broader adoption of follicular ultrasonography in sheep. Devices with rezoluție mai mare, multiple grayscale levels, and extended battery life are becoming more accessible to medium- and small-scale farmers.
In the United States, large sheep breeding operations often pair reproductive ultrasound with hormone assays (like progesterone or estradiol testing) to provide a complete picture of the ewe’s reproductive status. In Brazil and Argentina, B-mode ultrasound is integrated into superovulation and embryo transfer protocols, helping breeders improve genetic selection.
Concluzie
Ultrasonography has revolutionized how sheep producers and veterinarians manage and assess follicular development during superovulation. By understanding the image characteristics—anechoic fluid areas with bright echogenic rims—and identifying the optimal window (Day 3–4 post-superovulation treatment), livestock professionals can greatly enhance reproductive efficiency.
This approach not only improves the timing of insemination and embryo recovery but also reduces economic losses associated with poor superovulatory responses. As ultrasound technology continues to evolve and become more farmer-friendly, its role in precision sheep reproduction is expected to grow.
For breeders aiming to maximize genetic gains and reproductive outcomes, incorporating routine follicular ultrasonography is not just a recommendation—it is becoming a necessity.
References
-
González-Bulnes, A., Menchaca, A., & Bó, G. (2021). Reproductive Technologies in Sheep and Goats. Veterinary Reproduction Advances, 18(3), 142-158.
-
Whitaker, D. A., & Smith, E. (2021). Veterinary Ultrasonography in Food-Producing Animals. Journal of Veterinary Imaging.
-
Beef Cattle Institute. (2023). “Use of Ultrasound for Growth Evaluation in Cattle.” https://www.beefcattleinstitute.org/ultrasound-growth
-
University of Saskatchewan. (2020). “Follicular Dynamics and Superovulation in Small Ruminants.” https://research.usask.ca/ovarian-monitoring